ACUTE SPINAL CORD INJURY (ALMB-0166)
Acute spinal cord injury (SCI) results from a fracture or dislocation of the vertebrae and injured spinal cords and it results in a profound, negative impact on the patient’s physical, neurological, psychological, and psychosocial well-being. Acute SCI affects an estimated 17,000 people per year in the United States, many of them are young adults who must deal with the physical and neurological deficits resulting from their injury for many decades.

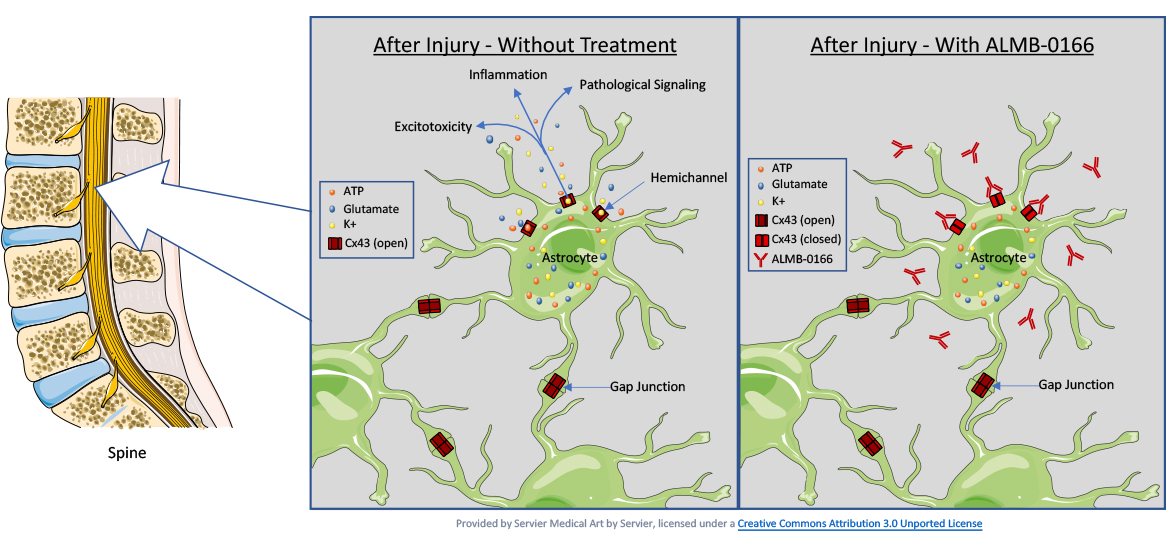
Negative neurological effects caused by acute SCI involve a complex of primary and secondary damage due to the release of pro-inflammatory molecules. The post-injury neuroinflammation results in the formation of a scar that prevents the neuronal axons from regenerating to restore neurological function.
Connexin 43 hemichannels in astrocytes open as a result of the injury, release pro-inflammatory molecules, and result in harmful inflammation. ALMB-0166 inactivates the connexin43 hemichannels causing them to close. This prevents the release of the pro-inflammatory molecules and results in reducing the local inflammation and scar formation, allowing the neurons to regenerate and improving the recovery from spinal cord injury.
AlaMab would welcome the opportunity to discuss possible investment or partnering opportunities.
